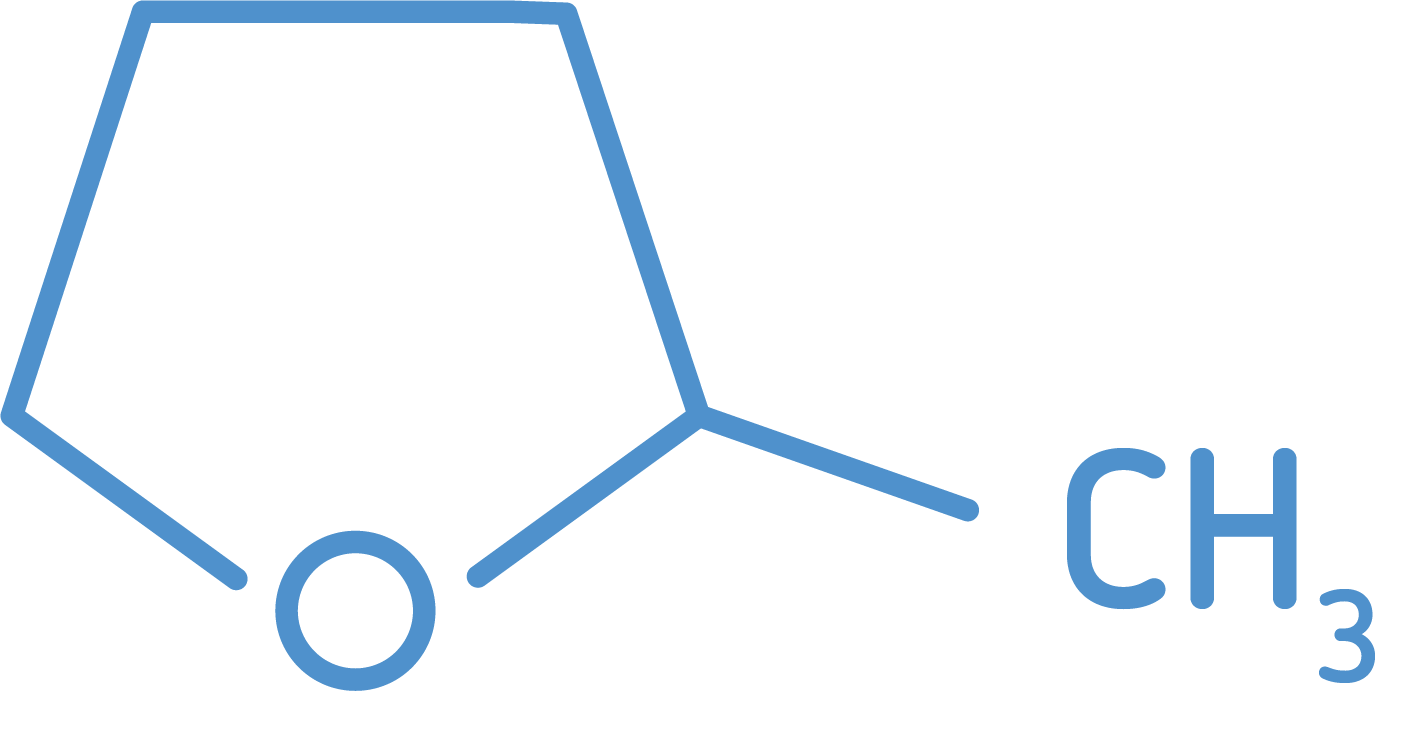
2-MeTHF
Monument Chemical produces high-quality 2-Methyltetrahydrofuran (2-MeTHF) at our Bayport, Texas facility. Leading pharmaceutical and fine chemical companies around the world have embraced 2-MeTHF as a green solvent with unique properties and performance advantages over alternative products.
2-MeTHF Features & Applications:
- Organometallic Reactions
- Grignard Reactions
- Extraction Solvent
- Hydrogenation
- Alternative to Dichoromethane for biphasic reactions
THE MONUMENT ADVANTAGE
• Broad portfolio of fine chemicals & pharma solvents
• Strong focus on safety, reliability, and quality
• Highly responsive to customer needs
.png)
2-Methyltetrahydrofuran Physical Properties and Advantages
Physical Properties
MP = -136°C
BP = 80°C
MW = 86.13 g/mole
Formula = C5H10O
Density@20°C = 0.86 g/ml
Regulatory Toxicity
ACGIH = Not listed
CA Prop 65 = Not listed
LC50 (inhalation, rat) = 6,000 ppm (4 H)
LD50 (skin, rabbit) = 4,500 mg/Kg
EINECS Number = 202-507-4
Typical Properties
Assay = 99.9%
Appearance = Clear and free
Water = 300 ppm, max
BHT = 150-400 ppm
Click Here For more information.
Extraction Solvent
By using 2-MeTHF as an extraction solvent instead of THF or dichloromethane,you can take advantage of the low water solubility, higher extraction efficiency, clean phase separation, and acid/base stability to increase your isolated yields and simplify your downstream product isolation.
Grignard Reactions
The use of 2-MeTHF as a reaction solvent in Grignard reactions yields important benefits, as the formation of allylic and benzylic Grignards proceeds with a higher yield and with a reduced level of the undesired forming of Wurtz coupling by-product.1 Not only has it been found that 2-MeTHF diminishes undesired Wurtz coupling in the Grignard reaction, but it has also been shown that nickel catalyzed cross-coupling of aryl Grignards in 2-MeTHF can be carried out in good yields.2
1 Grignard Reaction in Mixed Solvent Systems, from CHI’s Process R&D Summit, Philadelphia, PA (October 4-6, 2006).
2 Zhenhua Li, et. al., Synthesis of 2, 4-diarylquinolines: nickel catalyzed ligand -free cross-couplings of 4-chloro-2-arylquinolines with arylmagnesium halides in 2-methyltetrahydrofuran. Journal of Chemical Research, 35 (4) 240-242 (2011).
Organometalic Reactions
An additional advantage of using 2-MeTHF in organometallic-based reactions is the improved stability of n-butyllithium in 2-MeTHF versus in THF. The half-life of n-butyllithium at 35ºC was measured as 70 minutes in 2-MeTHF, while only 10 minutes in THF.
Hydrogenation
Reduction of a compound (I) in a 2-MeTHF solution in the presence of MgCl gave an 85% average yield of the desired product (II) over an 18-batch campaign. Conversely, the same reaction using THF and MgBr proved problematic on scale-up due to side reac-tions of the product (II). 3 Terence Connolly, et. al., Modified Chelation-Controlled Reduction of an N-Acryloyloxazolidin -2-one, from Organic Process Research & Development, 14 (6), 1456-1460 (2010).



